[This article is the original article of Lefeng RephiLe company, please indicate the reprint, offenders. 】             In vitro diagnostic reagents are part of medical devices and include detection reagents, kits, calibrators, controls, etc., which can be used alone or in combination with instruments, appliances, equipment or systems. In the prevention, diagnosis, treatment monitoring, prognosis of, state of health evaluation process of genetic diseases and predicting the in vitro diagnostic reagents in vitro assays (In Vitro for human samples (various body fluids, cells, tissue samples, etc.) in Diagnosis , abbreviated as IVD) plays a key role. According to the "In vitro Diagnostic Reagent Registration Management Measures", in vitro diagnostic reagents are classified into the first category, the second category, and the third category. Different levels of in vitro diagnostic reagents have different levels of risk, with the third category being the highest. The State Food and Drug Administration issued the “ YiT 1244-2014 Purified Water for In Vitro Diagnostic Reagents †in 2014, clearly indicating the requirements for the use of purified water in vitro diagnostic reagents in the pharmaceutical industry: * Total organic carbon and easy oxide alternative detection Purified water for in vitro diagnostic reagents is equivalent to several grades of water in GB GB2? What is the difference between purified water for in vitro diagnostic reagents compared to the Chinese Pharmacopoeia (2015 edition)? What kind of pure water should I use for the production of colloidal gold? In addition to the potential to change the pH, organic matter forms a combination with proteins. Or unstable, easy to inactivate, or affect the sensitivity of the probe. Preparation of interfering protein-gold complexes. What are the requirements for in vitro diagnostic reagents for purified water manufacturers? What kind of service can Shanghai Kefeng provide for colloidal gold users? Provide users with bilingual manuals and services in Chinese and English , high quality technical and maintenance support. A complete solution for in vitro diagnostic reagents is available based on the specific needs of the user . The full range of formula filler column - low TOC type, low magnesium type, low boron type, ICP type, etc., to meet the needs of users. RephiBio terminal filter for the preparation of ultra-pure water without pyrogen (endotoxin), nuclease and bacteria for biochemical analysis and molecular biology. About Shanghai Lefeng Biotechnology Co., Ltd. Shanghai Lefeng Bio is specialized in the research, development, design and manufacture of high-end water purification and laboratory separation and purification products, and is committed to providing cutting-edge, high value-added innovative products for life sciences and biotechnology. The Lefeng product line includes laboratory pure water systems, Millipore pure water compatible consumables and laboratory separation and purification filtration products. Established ten years ago, Lefeng has established its own brand RephiLe (Ruifeng), with more than 30 patents and multiple software copyrights. Products are sold to nearly 90 countries and regions around the world. For more information on RephiLe products, please visit: Le Feng official website Pay attention to RephiLe Enterprise WeChat: Le Feng pure water, pay attention to Le Feng dynamic! Key words: pure water, in vitro diagnostic reagent, colloidal gold, Elisa, UVD, molecular diagnosis, POCT, Shanghai Lefeng, RephiLe, Genie water purifier, Super-Genie water purifier, Chinese Pharmacopoeia, laboratory, purified water, injection Water, Sterilized Water for Injection, Standard, Deionized Water, DI Water, Distilled Water, Reverse Osmosis Water, RO Water, Ultrapure Water, EDI, Filtration, Needle, Sterile, HPLC, ICP-MS, AAS, LC-MS , biochemical analyzer sequencing, NGS high-throughput sequencing, clinical, aging machine, bottle washer, ion chromatography, membrane, air filtration, carbon dioxide incubator, fermenter Sodium Methoxide CAS No.124-41-4 Sodium Methoxide Physicochemical Properties
show. Store and transport according to flammable chemicals.
Sodium Methoxide Application
1. Mainly used as raw materials for medicines and pesticides, also used in dyes and chemical fiber industries. Sodium Methoxide,Sodium Methoxide Msds,Sodium Methoxide Solution,Sodium Methoxide Formula,Sodium Methoxide Density,Sodium Methoxide Reaction Shandong YingLang Chemical Co.,Ltd , https://www.sdylhgtrade.com
Abstract: In vitro diagnostic reagents play a key role in the in vitro diagnosis (In Vitro Diagnosis, IVD) of human samples (various body fluids, cells, tissue samples, etc.), and the purified water used also plays an important role. . 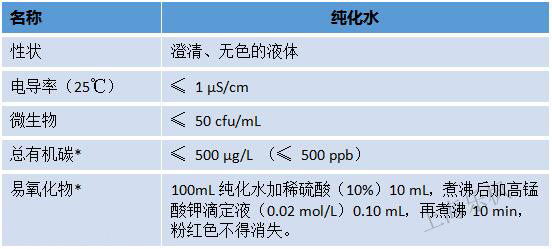
From the perspective of water quality, purified water for in vitro diagnostic reagents is equivalent to the secondary water of GB6642.
For the production of reagents with special requirements, the quality of purified water should have higher requirements. For example, molecular biology reagents have high requirements for DNAse, RNAse, and pyrogens.
1. Significantly reduced testing items The YY/T1244-2014 standard has reduced six items (pH, nitrate nitrite, ammonia, non-volatiles, heavy metals) compared to the Chinese Pharmacopoeia (2015 version ) . In addition to the pH measurement using pH meter, other projects use visual colorimetric methods. For non-pharmaceutical manufacturers, especially IVD companies, the daily laboratory preparation solution, colorimetric analysis test ability and professional The drug analysis laboratories are far apart, and such a large-scale elimination of testing programs is undoubtedly good news for IVD manufacturers.
2. Different methods for detecting oxides YY/T 1244-2014 standard stipulates that total organic carbon and easy oxides are optional . The Chinese Pharmacopoeia (2015 edition) stipulates that the total organic carbon test method should use the total organic carbon tester.
3. Different conductivity requirements After conversion, we confirm the conductivity of purified water specified by YY/T1244-2014 (25 °C) ≤ 1 μS/cm; and the conductivity of purified water specified in the Chinese Pharmacopoeia (2015 edition) (25) °C) ≤ 5.1 μS/cm. The former is equivalent to the secondary water of the national standard GB6682, and the latter is equivalent to the third-grade water. The conductivity of YY/T1244 is higher .
4. Different microbial requirements YY/T 1244-2014 stipulates that microorganisms ≤ 50 cfu/mL; Chinese Pharmacopoeia (2015 edition) stipulates that: microorganisms ≤ 100 cfu/mL. The microbiological requirements of YY/T1244 are higher .
Colloidal gold is polymerized into a certain size of gold particles by chloroauric acid under the action of a reducing agent (such as white phosphorus, ascorbic acid, sodium citrate, etc.), and is electrostatically stabilized into a stable colloidal state.
Ions change the pH and ionic strength. The pH has a certain effect on the final particle size. The color of the solution is related to its pH. The ionic strength affects the successful combination of colloidal gold and protein, or leads to the aggregation of colloidal gold particles and reduces the detection ability of colloidal gold. 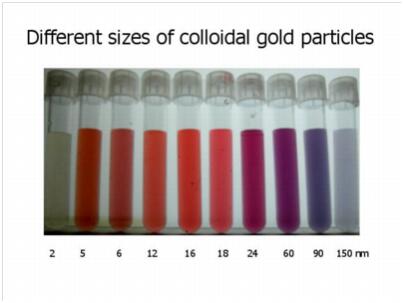
The particles interfere with the formation of colloidal gold particles, forming particles of varying sizes, reddish, colorless or opaque.
Therefore, the preparation of colloidal gold must use pure water with low ion content, low TOC and no particles.
According to the Regulations for the Production of In Vitro Diagnostic Reagents, the water-making equipment should meet the water quality requirements and pass the verification. Therefore, pure water equipment manufacturers must provide 3Q verification services to help in vitro diagnostic reagent manufacturers pass GMP verification. Lefeng can provide users with bilingual manuals and services in Chinese and English, high-quality technical and maintenance support to ensure you have no worries about water. 
Density 0.97 g/mL at 20 °C
Boiling point 65 °C
Melting point -98 °C
Molecular formula CH3NaO
Molecular weight 54.024
Flash point 11 °C
Exact quality 54.008160
PSA 23.06000
LogP 0.04670
Appearance traits transparent liquid
Vapor density 1.1 (vs air)
Steam pressure 50 mm Hg ( 20 °C)
Refractive index 1.3700
Storage Conditions
1. Storage: sealed and stored in a cool, dry and dark place
2. Sealed in iron drums, 200kg per barrel, stored in
Cool, ventilated, dry place, fireproof, heatproof, prevent
2. It is used as a condensing agent in organic synthesis, as a catalyst in the treatment of edible oils and fats, and as an important raw material for the synthesis of drugs such as sulfamididine, sulfamethoxazole and sulfonamide synergist.
3. Used as an alkaline condensing agent and catalyst in organic synthesis, used in the synthesis of perfumes, dyes, etc., and is a raw material for vitamins B1, A and sulfadiazine.
4. Used as a condensing agent for organic synthesis
5. Fat transesterification catalyst. To change the fat structure, make it suitable for margarine and so on. Must be removed in the final food.
6, mainly used as a condensing agent, strong alkaline catalyst and methoxylation agent, used to prepare vitamin B1 and A, sulfadiazine and other drugs, a small amount for pesticide production. It is also used as a catalyst for the treatment of edible fats and edible oils, especially lard. Also used as an analytical reagent.
7, mainly used in the production of vitamin A1 vitamin B1 long-acting sulfonamide, sulfadiazine, trimethoprim and other pharmaceutical industries, can also be used in the biodiesel industry. It can also be used as an edible catalyst and analytical reagent.
8, mainly used as a condensing agent; strong alkaline catalyst and methoxylation agent, used to prepare vitamins B1 and A; sulfadiazine and other drugs, a small amount for pesticide production. It is also used as a catalyst for the treatment of edible fats and edible oils, especially lard. Also used as an analytical reagent.