Simultaneous activation of innate & adaptive immunity! The new immune combination NKRT-262/NKTR-214 demonstrates the therapeutic potential of multiple types of cancer March 06, 2019 Source: Bio Valley Nektar Therapeutics is a clinically based biopharmaceutical company dedicated to the discovery and development of innovative drugs to meet the unmet medical needs of patients. The company's pipeline includes assets to treat cancer, autoimmune diseases and chronic pain. Recently, the company announced the new immunological oncology drug NKRT-262 combined with bempegaldesleukin (bempeg, NKTR-214) in the 2019 ASCO-SITC clinical immunooncology seminar in San Francisco, USA. Study the early results of REVEAL (NCT03435640). Dear ladies, are you confused on the road to health and beauty? Our company brings you a health product specially designed for modern women, so that you can feel confident and show your most beautiful self! Whether it`s busy work or stressful life, we all need to take care of our bodies and minds. Let`s explore this exciting new product together! menopause supplements,best supplements for weight loss female,best collagen supplement for women Xi'an complex bio-tech CO.,LTD. , https://www.complexpowder.com
NKRT-262 is a novel Toll-like receptor (TLR) 7/8 agonist designed to induce the body's innate immune response to mobilize antigen-specific cytotoxic T cells against cancer; bempegaldesleukin is a CD122 biased IL-2 Pathway agonists that aim to activate the adaptive immune system to amplify and propagate these specific anti-cancer T cells in the tumor microenvironment. 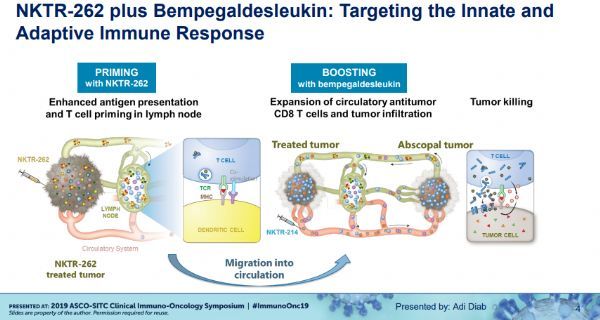
REVEAL is an open-label, multicenter, dose escalation, and dose-expanding study evaluating NKTR-262 as an initial intratumoral injection, followed by a combination of bempegaldesleukin as a systemic intravenous infusion (two) . In the phase II expansion phase, the study may also evaluate the combination of this two-way protocol with Opdivo (Oddi, Navumab) (triple). In the dose escalation phase, a recommended phase II dose (RP2D) regimen of two and/or triple combinations will be determined. The dose escalation phase will evaluate two and/or triple combinations in up to 350 patients diagnosed with locally advanced or metastatic cancer, including: melanoma, Merkel cell carcinoma, triple negative breast cancer, ovarian cancer, kidney Cell carcinoma, colorectal cancer, urothelial carcinoma or sarcoma.
The dose escalation phase of the REVEAL study is ongoing, and as of January 23, 2019, a total of 13 patients were included in the study, and these patients did not respond to all previous therapies known to have clinical benefit. Highlights highlighted at the meeting included: (1) The maximum tolerated dose has not been reached and the dose escalation phase of the study continues. (2) NKTR-262 intratumoral injection combined with a fixed dose of NKTR-214 (intravenous infusion [IV], once every 3 weeks [Q3W]) is well tolerated, and treatment-related adverse events are transient, including 1-2 Grade flu-like symptoms (69.2%), rash (46.2%), fatigue (46.2%), pruritus (46.2%), and nausea (30.8%), no immune-mediated adverse events or treatment-related adverse events (TRAE) Interrupted the study. (3) Local gene expression analysis of injected tumors and blood cell analysis in systemic circulation showed that the immune system was fully activated, including activation of the type I interferon pathway and induction of CD4+, CD8+ and NK cell proliferation.
(4) Early clinical benefit evidence was observed in the dose escalation cohort, including the distant response to uninjected tumors. Eleven of the 13 dose-increasing patients received at least one treatment scan to assess their efficacy. Of the 5 patients with relapsed/refractory (R/R) melanoma who had undergone more than one checkpoint or immuno-oncology (IO) therapy for disease progression, 2 patients were confirmed according to RECIST 1.1. Partial relief, target lesions were reduced by 100% and 50%, respectively. Two patients with over-treatment of stage IV leiomyosarcoma and one patient with over-treatment of triple-negative breast cancer experienced the best response to stable disease.
Dr. Jonathan Zalevsky, Chief Scientific Officer of Nektar, said, "We are excited about the preliminary data from the REVEAL study, which demonstrates the predictable changes in the tumor microenvironment and the activation of innate and adaptive immune responses induced by NKTR-262 and bembegaldesleukin. Consistent with these early data, a comprehensive approach that activates the body's immune system can drive distant anti-tumor responses even when checkpoint inhibitors are not used."
Original Source: Nektar Therapeutics Presents Preliminary Immune Activation, Safety and Clinical Activity Data from the Ongoing Dose-Escalation Stage of the REVEAL Study at 2019 ASCO-SITC Meeting
Our feminine health products are carefully developed to meet the needs of the modern woman. It uses natural ingredients and is scientifically proportioned to help regulate female endocrine and improve problems such as irregular menstruation and premenstrual symptoms. Plus, it`s rich in Vitamins and antioxidants, which help improve skin`s radiance and delay signs of aging, leaving you with a youthful glow.
What sets our women's health products apart from other products on the market is that we understand women's needs and concerns. Our products undergo strict quality control to ensure that each product meets the highest quality standards. Additionally, our formulas have been researched and proven by a team of professionals to help women better cope with life's stresses and challenges.
Thousands of women have experienced and benefited from our products. They find that their bodies are healthier, their moods are happier and their skin is brighter when they use our women's supplements. Let`s listen to their voices: [This product is great! I feel rejuvenated and my work efficiency has improved!", [Taking this health product every day, I no longer worry about irregular menstruation. , feeling relaxed and at ease." These real testimonials demonstrate the excellent effects and user satisfaction of our products.
Our company adheres to the customer-centered principle and is committed to providing you with the highest quality products and services. We promise that if you are not satisfied with our women's health products, we will give you a full refund. Our goal is for every female customer to enjoy the benefits of our products and feel satisfied and assured.
Take action now to rejuvenate your body and mind! You can purchase our women's health products through our official website, authorized dealers or offline stores. We offer a variety of purchasing methods and flexible payment methods to meet different needs. After purchase, we will ensure that the product is delivered in time and provide high-quality after-sales service.
Dear ladies, you deserve the best in life and health. Choosing our women's health products is a kind of care and investment in yourself. Let us work together towards a healthier and more beautiful future! Buy now, feel the difference and become your most beautiful self!