CAS No.461432-26-8
Chemical Name:Dapagliflozin
SynonymsBMS5121458;Bms 512148;DAPAGLIFLOZIN;BMS-512148-05;Dag coluMn net;Dapagliflozin, 99%;Dapagliflozin(BMS-512148);Dapagliflozin propanediol;Dapagliflozin Isomer Impurity;Dapagliflozin S1548 Selleck
CBNumber:CB91011730
Molecular Formula:C21H25ClO6
Formula Weight:408.875
Form:White Solid
Â
Product Name
Daggeregnet
CAS
461432-26-8
Standard
Enterprise Standard
Natural/Synthetic
Synthesis
Source of extraction
Synthesis
Level
Pharmaceutical Grade
Content
99%
Appearance
white powder
Packing
100G/aluminum foil bag
Ingredients
(1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol
Field
Intermediates
Deferred Products
Dapagliflozin (S)-Propylene Glycol Hydrate
Application
Dapagliflozin (S)-propylene glycol hydrate intermediate
Diabetes drugs
Dapagliflozin (ForxigaTM) is a new antidiabetic drug jointly developed by Bristol-Myers Squibb and AstraZeneca, being approved by the European Medicines Agency (EMA) on November 12, 2012. It is also the first approved SGLT2 inhibitor for the treatment of type II diabetes, being an important option in the treatment of diabetes, and is used to improve glycemic control as an adjunct to dietary and exercise for adults with type II diabetes.
Dapagliflozin is a sodium-glucose co-transporter 2 inhibitor. On January 8, 2014, the US Food and Drug Administration (FDA) have approved it for being used in the treatment of type II diabetes. Meanwhile, FDA requires the producers to conduct post-marketing research on drug-related risks.
The post-marketing trial requested by the FDA includes a cardiovascular outcome trial for assessing the cardiovascular risk for high-risk patients after treatment with dapagliflozin at baseline and a study to assess the risk of bladder cancer in recruited patients. Another study will assess the bladder tumor-promoting effect of this drug on rodent animals. Two studies will assess the pharmacokinetics, efficacy and safety of dapagliflozin in pediatric patients; a set of strengthened pharmacovigilance program will monitor liver abnormalities and pregnancy outcome reports in patients receiving daglitazone.Â
Pharmacological effects
Dapagliflozin works through inhibiting sodium-glucose transporter 2 (SGLT2), a protein in the kidney that reabsorbs glucose into the bloodstream. This allows extra glucose to be excreted through the urine, improving glycemic control without increasing insulin secretion. The use of this drug requires patients with normal renal function while patients of moderate to severe renal insufficiency should be disabled to use this drug. Single application of this product or combination with metformin, pioglitazone, glimepiride, insulin and other drugs can significantly reduce the HbA1c and fasting blood glucose of patients suffering type II diabetes. The frequency of the adverse reaction was similar to placebo with low risk of hypoglycemia, being able to reduce body weight.
The efficacy of dapagliflozin is comparable with the dipeptidyl peptidase inhibitors, and several new hypoglycemic drugs, and can also mildly lower the blood pressure and body weight. The drug has 5mg and 10mg two tablets to choose from, can be either used alone or together with insulin, including other diabetes drugs.
Pharmacokinetics
In healthy subjects, dapagliflozin was rapidly absorbed after oral administration with a peak time Tmax being 1 to 2 hours, a protein binding rate of 91%, an oral bioavailability of about 78% and a plasma terminal half-life of 12.9 hours. After oral administration, the drug is mainly metabolized by the uridine diphosphate glucuronosyltransferase 1A9 (UGT1A9) into the inactive metabolite in the liver with the smaller part being metabolized by the P450 enzyme and of no inhibitory or inducing effect on the P450 enzyme. Drug prototypes and related metabolites were excreted through urine (75%) and faeces (21%). Compare simultaneous administration of this product with high-fat food and with the fasting administration, Tmax can be extended by 1-fold, but the absorption did not affect the degree, so can be administrated together with the food.
The pharmacokinetics of daglitazone was significantly affected by renal function. Diabetic patients with mild, moderate or severe renal insufficiency are merged to be subject to oral administration of 20 mg • d-1 daglitazone for 7 days. The mean systemic exposure amount, compared with patients with normal renal function, is respectively 32%, 60% and 87% higher. For patients with normal renal function, mild insufficiency, moderate insufficiency and severe insufficiency, the urinary glucose excretion amount in 24 hours of steady state was 85, 52, 18 and 11g, successively.
Kasichayanula et al have studied the pharmacokinetic effects of liver dysfunction on daglitazone. The patients with mild, moderate and severe hepatic insufficiency having a single oral dose of 10 mg of daglitazone, the Cmax of each group was 12% lower, 12% higher and 40% higher than that with normal liver function, respectively. The AUC of each group was significantly higher than that of normal liver function by 3%, 36% and 67%.
Therefore, it is not recommended to apply daglitazone to patients of moderate and severe renal dysfunction. Severe liver dysfunction patients need to reduce the use of dose.
Synthesis method
5-bromo-2-chlorobenzoic acid is subject to acylating chlorination, and has Friedel-Crafts reaction with phenylethyl ether for reduction of its carbonyl group, generating 5-bromo-2-chloro-4'-ethoxydiphenyl methane, further subjecting to condensation with 2, 3, 4, 6-tetra-O-trimethylsilyl-D-glucopyranosanoic acid-1,5-lactone. The anomeric carbon hydroxyl group is subject to etherification and deprotection to give 2-chloro-5-(1-methoxy-D-glucopyranose-I-yl)-4'-ethoxydiphenylmethane, and then use Et3SiH/BF3 • OEt2 for reduction to remove methoxy, followed by acetic anhydride esterification and hydrolysis to give hypoglycemic agents daglitazone with the overall yield of about 40%.
Our advantages:
A. Saichuang Technology Co., ltd is a professional raw powder factory in China for over 10 years, all powders are factory directly supplying.
B. Our products have exported to Germany, Norway, Poland, Finland, Spain, UK, France, Russia, USA, Australia, Japan, Korea and many other countries, over 100kgs each month.
C. Professional team special for package and shipment and staring on tracking code 24hours for customs pass guaranteed. 100% pass to UK, Norway, Poland, Spain, USA, Canada, Brazil; 98% pass to Germany, Russia, Australia, New Zealand.
D. Most of powders are in stock, Chargeable samples are available, Could be shipped out within 24hours.
E. High quality, good price, fast and safety delivery. Shipment by DHL, TNT, FEDEX, HKEMS, UPS, etc.
FAQ
Order Guide
Q1: Have your Product Quality been Approved by Third Party Lab?
A: Yes, All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France etc. So you will be assured with Good Quality if you choose us.Â
Â
Q2:How do you treat quality complaint?
A:First of all, our QC department will do strict examination of our export products by HPLC, UV, GC ,Â
TLC and so on in order to reduce the quality problem to near zero. If there is a real quality problem ,caused by us, we will send you free goods for replacement or refund your loss.
Â
Q3: Do you Accept Sample Order?
A: Yes, we accept small order from 10g, 100g and 1kg for your evaluation quality of our goods.
Q4: Is there any discount?
A: Yes, for larger quantity, we always support with better price.Â
Q5:Do you accept VISA business credit card ?
A:Sorry we don't accept VISA credit card,
we'd like to accept bank transfer, Western Union or Paypal or  Moneygram
Q6:How long does it take to the goods arrived ?
A:It is Depending on your location,
    For small order, please expect 5-7 days by DHL,UPS,TNT, FEDEX, EMS.
For mass order, please allow 5-8 days by Air, 20-35 days by Sea.
Q7  Do  you  have  any  reshipment  policy ? Â
we have good after-sale service and re-shipment policy if the parcel  lose
Our long association with  our clients has brought great benefits
We always take the upmost care in the packaging of our productsÂ
our clients will confirm this as even they struggle to find them without help at times.
But in spite of our best efforts it is still possible  will seize a small number of packages.
In this circumstance we promise reship free  to establish  long term relationship
Â
Q8 :Can I get a sample?
A: Of course. For most products we can provide you a free sample, while the shipping cost should undertake by your side.
We specialized in manufacturing high quality and reliable raw materialÂ
which are widely used in the fields of food, health, cosmetics, pharmaceuticals, and Bodybuilding
Â
we have formed a technology and management team that cooperate closely with some  overseas Labs  all speak highly of our safe shipping and goods .
please donot hesitate to contact us.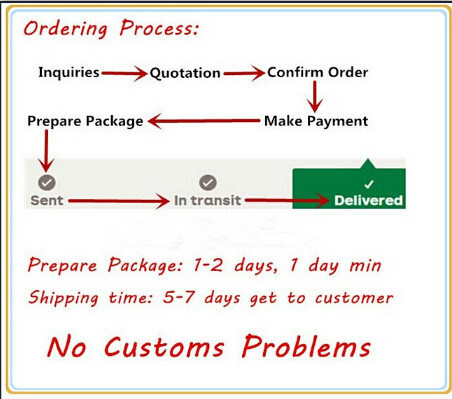
If you have any further query,please feel free to let me know.
Zoey Li
XiamenShengLangSaiChuangBiologicalTechnologyCo.,?Ltd
Â
Â
Â
Product description:
CAS No.461432-26-8
Chemical Name:Dapagliflozin
SynonymsBMS5121458;Bms 512148;DAPAGLIFLOZIN;BMS-512148-05;Dag coluMn net;Dapagliflozin, 99%;Dapagliflozin(BMS-512148);Dapagliflozin propanediol;Dapagliflozin Isomer Impurity;Dapagliflozin S1548 Selleck
CBNumber:CB91011730
Molecular Formula:C21H25ClO6
Formula Weight:408.875
Form:White Solid
Â
Product Name
Daggeregnet
CAS
461432-26-8
Standard
Enterprise Standard
Natural/Synthetic
Synthesis
Source of extraction
Synthesis
Level
Pharmaceutical Grade
Content
99%
Appearance
white powder
Packing
100G/aluminum foil bag
Ingredients
(1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol
Field
Intermediates
Deferred Products
Dapagliflozin (S)-Propylene Glycol Hydrate
Application
Dapagliflozin (S)-propylene glycol hydrate intermediate
Diabetes drugs
Dapagliflozin (ForxigaTM) is a new antidiabetic drug jointly developed by Bristol-Myers Squibb and AstraZeneca, being approved by the European Medicines Agency (EMA) on November 12, 2012. It is also the first approved SGLT2 inhibitor for the treatment of type II diabetes, being an important option in the treatment of diabetes, and is used to improve glycemic control as an adjunct to dietary and exercise for adults with type II diabetes.
Dapagliflozin is a sodium-glucose co-transporter 2 inhibitor. On January 8, 2014, the US Food and Drug Administration (FDA) have approved it for being used in the treatment of type II diabetes. Meanwhile, FDA requires the producers to conduct post-marketing research on drug-related risks.
The post-marketing trial requested by the FDA includes a cardiovascular outcome trial for assessing the cardiovascular risk for high-risk patients after treatment with dapagliflozin at baseline and a study to assess the risk of bladder cancer in recruited patients. Another study will assess the bladder tumor-promoting effect of this drug on rodent animals. Two studies will assess the pharmacokinetics, efficacy and safety of dapagliflozin in pediatric patients; a set of strengthened pharmacovigilance program will monitor liver abnormalities and pregnancy outcome reports in patients receiving daglitazone.Â
Pharmacological effects
Dapagliflozin works through inhibiting sodium-glucose transporter 2 (SGLT2), a protein in the kidney that reabsorbs glucose into the bloodstream. This allows extra glucose to be excreted through the urine, improving glycemic control without increasing insulin secretion. The use of this drug requires patients with normal renal function while patients of moderate to severe renal insufficiency should be disabled to use this drug. Single application of this product or combination with metformin, pioglitazone, glimepiride, insulin and other drugs can significantly reduce the HbA1c and fasting blood glucose of patients suffering type II diabetes. The frequency of the adverse reaction was similar to placebo with low risk of hypoglycemia, being able to reduce body weight.
The efficacy of dapagliflozin is comparable with the dipeptidyl peptidase inhibitors, and several new hypoglycemic drugs, and can also mildly lower the blood pressure and body weight. The drug has 5mg and 10mg two tablets to choose from, can be either used alone or together with insulin, including other diabetes drugs.
Pharmacokinetics
In healthy subjects, dapagliflozin was rapidly absorbed after oral administration with a peak time Tmax being 1 to 2 hours, a protein binding rate of 91%, an oral bioavailability of about 78% and a plasma terminal half-life of 12.9 hours. After oral administration, the drug is mainly metabolized by the uridine diphosphate glucuronosyltransferase 1A9 (UGT1A9) into the inactive metabolite in the liver with the smaller part being metabolized by the P450 enzyme and of no inhibitory or inducing effect on the P450 enzyme. Drug prototypes and related metabolites were excreted through urine (75%) and faeces (21%). Compare simultaneous administration of this product with high-fat food and with the fasting administration, Tmax can be extended by 1-fold, but the absorption did not affect the degree, so can be administrated together with the food.
The pharmacokinetics of daglitazone was significantly affected by renal function. Diabetic patients with mild, moderate or severe renal insufficiency are merged to be subject to oral administration of 20 mg • d-1 daglitazone for 7 days. The mean systemic exposure amount, compared with patients with normal renal function, is respectively 32%, 60% and 87% higher. For patients with normal renal function, mild insufficiency, moderate insufficiency and severe insufficiency, the urinary glucose excretion amount in 24 hours of steady state was 85, 52, 18 and 11g, successively.
Kasichayanula et al have studied the pharmacokinetic effects of liver dysfunction on daglitazone. The patients with mild, moderate and severe hepatic insufficiency having a single oral dose of 10 mg of daglitazone, the Cmax of each group was 12% lower, 12% higher and 40% higher than that with normal liver function, respectively. The AUC of each group was significantly higher than that of normal liver function by 3%, 36% and 67%.
Therefore, it is not recommended to apply daglitazone to patients of moderate and severe renal dysfunction. Severe liver dysfunction patients need to reduce the use of dose.
Synthesis method
5-bromo-2-chlorobenzoic acid is subject to acylating chlorination, and has Friedel-Crafts reaction with phenylethyl ether for reduction of its carbonyl group, generating 5-bromo-2-chloro-4'-ethoxydiphenyl methane, further subjecting to condensation with 2, 3, 4, 6-tetra-O-trimethylsilyl-D-glucopyranosanoic acid-1,5-lactone. The anomeric carbon hydroxyl group is subject to etherification and deprotection to give 2-chloro-5-(1-methoxy-D-glucopyranose-I-yl)-4'-ethoxydiphenylmethane, and then use Et3SiH/BF3 • OEt2 for reduction to remove methoxy, followed by acetic anhydride esterification and hydrolysis to give hypoglycemic agents daglitazone with the overall yield of about 40%.
Our advantages:
A. Saichuang Technology Co., ltd is a professional raw powder factory in China for over 10 years, all powders are factory directly supplying.
B. Our products have exported to Germany, Norway, Poland, Finland, Spain, UK, France, Russia, USA, Australia, Japan, Korea and many other countries, over 100kgs each month.
C. Professional team special for package and shipment and staring on tracking code 24hours for customs pass guaranteed. 100% pass to UK, Norway, Poland, Spain, USA, Canada, Brazil; 98% pass to Germany, Russia, Australia, New Zealand.
D. Most of powders are in stock, Chargeable samples are available, Could be shipped out within 24hours.
E. High quality, good price, fast and safety delivery. Shipment by DHL, TNT, FEDEX, HKEMS, UPS, etc.
FAQ
Order Guide
Q1: Have your Product Quality been Approved by Third Party Lab?
A: Yes, All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France etc. So you will be assured with Good Quality if you choose us.Â
Â
Q2:How do you treat quality complaint?
A:First of all, our QC department will do strict examination of our export products by HPLC, UV, GC ,Â
TLC and so on in order to reduce the quality problem to near zero. If there is a real quality problem ,caused by us, we will send you free goods for replacement or refund your loss.
Â
Q3: Do you Accept Sample Order?
A: Yes, we accept small order from 10g, 100g and 1kg for your evaluation quality of our goods.
Q4: Is there any discount?
A: Yes, for larger quantity, we always support with better price.Â
Q5:Do you accept VISA business credit card ?
A:Sorry we don't accept VISA credit card,
we'd like to accept bank transfer, Western Union or Paypal or  Moneygram
Q6:How long does it take to the goods arrived ?
A:It is Depending on your location,
    For small order, please expect 5-7 days by DHL,UPS,TNT, FEDEX, EMS.
For mass order, please allow 5-8 days by Air, 20-35 days by Sea.
Q7  Do  you  have  any  reshipment  policy ? Â
we have good after-sale service and re-shipment policy if the parcel  lose
Our long association with  our clients has brought great benefits
We always take the upmost care in the packaging of our productsÂ
our clients will confirm this as even they struggle to find them without help at times.
But in spite of our best efforts it is still possible  will seize a small number of packages.
In this circumstance we promise reship free  to establish  long term relationship
Â
Q8 :Can I get a sample?
A: Of course. For most products we can provide you a free sample, while the shipping cost should undertake by your side.
We specialized in manufacturing high quality and reliable raw materialÂ
which are widely used in the fields of food, health, cosmetics, pharmaceuticals, and Bodybuilding
Â
we have formed a technology and management team that cooperate closely with some  overseas Labs  all speak highly of our safe shipping and goods .
please donot hesitate to contact us.
If you have any further query,please feel free to let me know.
Zoey Li
XiamenShengLangSaiChuangBiologicalTechnologyCo.,?Ltd
Â
Â
Â
High-Purity Competitive Dapagligin 99% CAS: 461432-26-8
Model NO.: 461432-26-8
Trademark: SL
Transport Package: Foil Bag
Specification: 100G/Foil Bag
Origin: China
HS Code: 6801000000
Model NO.: 461432-26-8
Trademark: SL
Transport Package: Foil Bag
Specification: 100G/Foil Bag
Origin: China
HS Code: 6801000000
Product description: