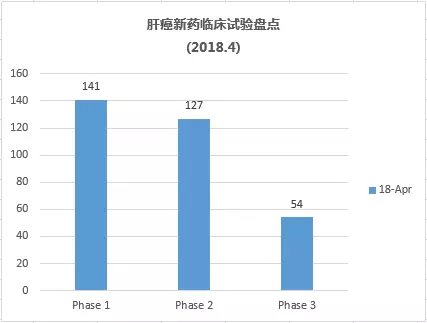
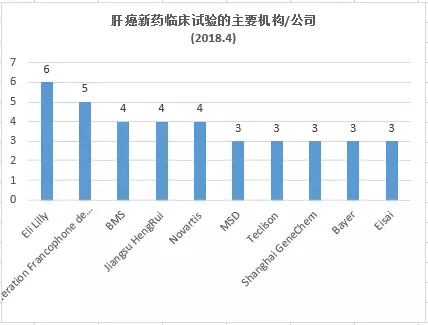
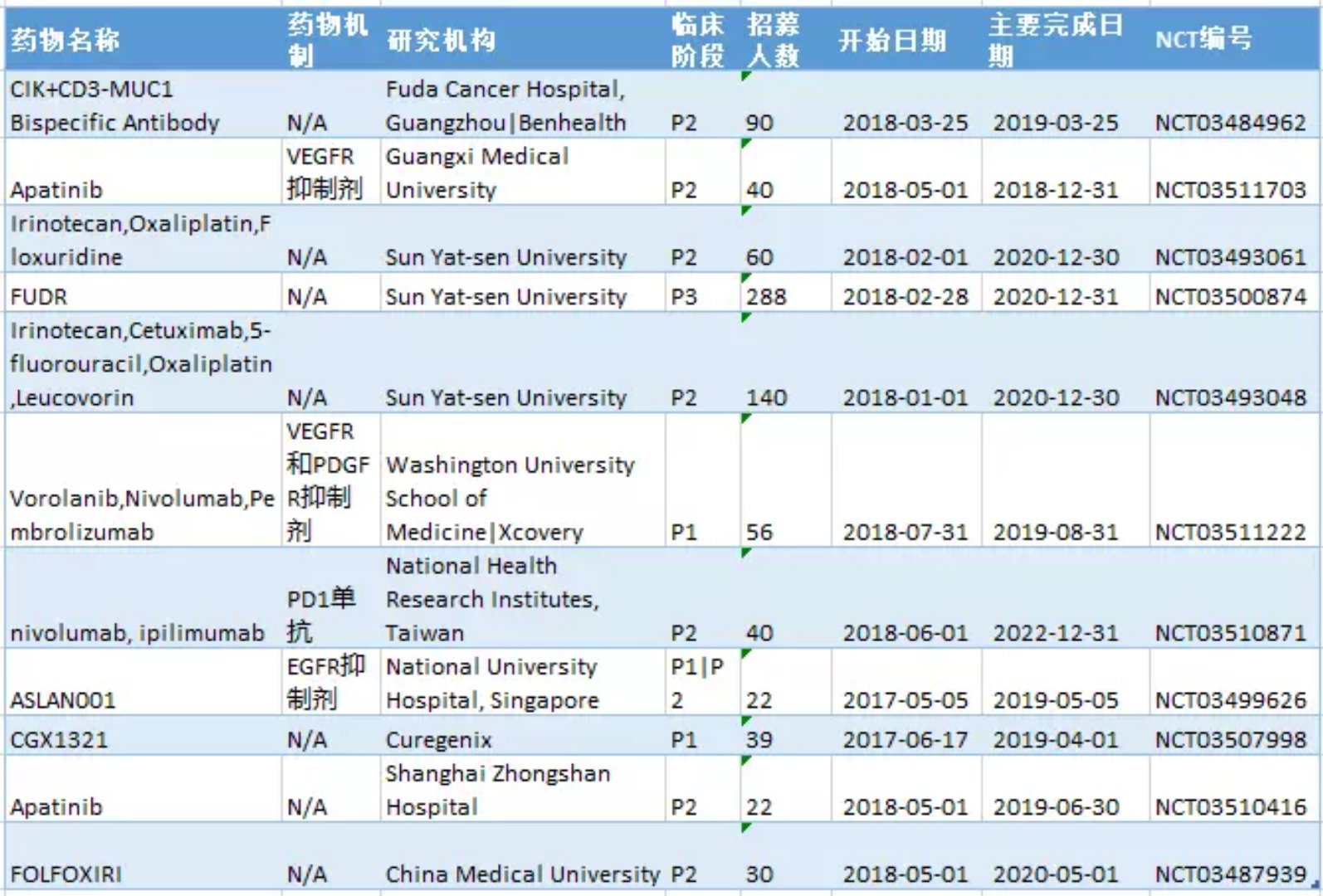
What are the latest liver cancer research and development pipelines in April 2018? May 15, 2018 Source: WuXi PharmaTech Primary liver cancer is the sixth most common cancer in the world (6%) and the second most common cancer. It is estimated that 750,000 people die of liver cancer every year worldwide. The main cause of liver cancer is cirrhosis caused by hepatitis B, hepatitis C or alcohol. The most common types of liver cancer are hepatocellular carcinoma (HCC) and cholangiocarcinoma, which account for about 80% of liver cancer. The area of ​​the disease varies widely, with approximately 80% of new cases occurring in Asia, including China and Japan. Liver cancer is a serious and high-risk cancer, but treatment options are very limited. Most systemic drugs are not effective in treating liver cancer. In November 2007, the FDA approved sorafenib for the treatment of advanced hepatocellular carcinoma. Sorafenib is a targeted drug that blocks cell proliferation and cell growth and provides survival benefits for patients with advanced liver cancer. The systemic treatment currently approved for first-line therapy for liver cancer is very limited and highlights this unmet medical need. ▲Little knowledge of liver disease (Source: 123RF) This article takes stock of the latest clinical development pipeline for liver cancer in April 2018. We calculated clinical trial data for clinical, phase 2, and phase 3 liver cancer drugs enrolled at clinicaltrials.gov. Included in the statistics are trials that are being recruited, recruited, and issued recruitment notices, and do not include trials that have been completed, terminated, unknown, withdrawn, and suspended. New drug pipeline inventory in the clinical stage of liver cancer As of April 30, 2018, there were 322 clinical trials of new drugs in the field of liver cancer, and early research was dominant. These include: 141 trials in Phase 1, including 104 small molecule drugs and 37 biopharmaceuticals; 127 trials in Phase 2, including 109 small molecule drugs and 18 biopharmaceuticals; 54 trials, including 48 small molecule drugs and 6 biological drugs (note: some drugs may be tested simultaneously). Clinical trials of new liver cancer drugs are concentrated in the United States and Asia. According to the statistics of the research institutes of clinical trials, the top 5 countries or regions are: 142 trials in the United States, 95 in mainland China, 68 in South Korea, 36 in Taiwan, and 36 in France (Note: There may be multiple centers) Clinical Trials). The research institutes/companies that carried out the largest number of clinical trials of new drugs for liver cancer were: Eli Lilly, 6 trials, 5 Federation Fedophone de Cancerologie Digestive companies, 4 Bristol-Myers Squibb, Jiangsu Hengrui, and Novartis, respectively, Merck, Teclison, Bayer, Eisai and Shanghai Jikai genes are three trials respectively. New clinical trial of new liver cancer drugs in April In April 2018, 11 new clinical trials of new liver cancer were added. An average of 22 subjects were recruited in one of the 1/2 trials, an average of 48 subjects were recruited in two phase 1 trials, an average of 60 subjects were recruited in 7 phase 2 trials, and an average of 288 trials were recruited in one phase 3 trial. Subject. ▲A new clinical study of new liver cancer drugs in April 2018 The following is a new clinical trial of liver cancer and an introduction of new drugs in April. New drug name: CIK and CD3-MUC1 Bispecific Antibody Drug mechanism: N/A Research institute: Guangzhou Fuda Cancer Hospital | Benkang Biopharmaceutical (Shenzhen) Co., Ltd. Clinical stage: Phase 2 This is a single-center, phase 2 immunotherapy clinical trial. The investigators plan to recruit 90 patients with advanced liver cancer. All patients were divided into three groups, one receiving cryotherapy, one receiving non-interventional therapy, and the remaining group receiving activated CIK and anti-CD3-MUC1 bispecific antibodies. Mixture and cryotherapy. The trial program enrolled 90 subjects. The expected study start date is March 25, 2018. The expected major completion date is March 25, 2019. New drug name: Apatinib (apatinib) and TACE treatment Drug Mechanism: VEGFR Tyrosine Kinase Inhibitor Research institution: Guangxi Medical University Clinical stage: Phase 2 Apatinib is a small molecule VEGFR tyrosine kinase inhibitor that treats malignant tumors mainly by inhibiting VEGFR and exerting anti-angiogenic effects. Apatinib has been shown to be safe and effective in patients with advanced gastric cancer who have failed standard chemotherapy. A randomized, double-blind, multicenter, phase 3 clinical trial of second-line treatment for advanced liver cancer has also shown good efficacy and safety. The TACE therapy map inhibits tumor growth and tumor atrophy by embolizing the blood supply to the tumor arteries. Based on the therapeutic potential of each of apatinib and TACED, the investigators designed a prospective clinical study of patients with advanced liver cancer. The purpose is to inhibit the growth of tumors, prolong the survival time of patients, and improve the quality of life as much as possible. Through treatment, patients have the opportunity to undergo surgical resection to more effectively extend the OS. The trial program enrolled 40 subjects. The expected start date for the study was May 1, 2018, and the expected major completion date was December 31, 2018. New drug name: Vorolanib, Nivolumab, Pembrolizumab Drug Mechanism: VEGFR and PDGFR Target Inhibitors + PD1 Monoclonal Antibody Research Institute: University of Washington School of Medicine | Xcovery Clinical stage: Phase 1 Vorolanib is an oral inhibitor of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) targets for the treatment of various cancers such as ophthalmology and kidney cancer, stomach cancer, and lung cancer. In 2017, Beida acquired 100% of Equinox's equity through acquisition and obtained the entire worldwide interest in vorolanib compound tumor indications. The investigators speculate that vorolanib combined with checkpoint inhibitors (pembrolizumab for gastric/gastroesophageal [GE] borderline cancer, nivolumab for hepatocellular carcinoma [HCC]) can overcome checkpoint inhibitors in gastrointestinal tumors (GI) Resistance, thereby improving immune efficacy. The trial plans to recruit 56 subjects. The expected start date for the study is July 31, 2018. The expected major completion date is August 31, 2019. New drug name: Nivolumab, Ipilimumab Drug mechanism: PD-1 mAb + CTLA-4 mAb Research Institute: Taiwan Institute of Health Research Clinical stage: Phase 2 The anti-PD-1 drug nivolumab has recently been approved by the US FDA for the treatment of patients with advanced hepatocellular carcinoma (HCC) who have received sorafenib. Preliminary data also show that the combination of nivolumab and anti-CTLA-4 therapy can improve the objective response rate of advanced HCC, and has better safety than conventional combination therapy (cytotoxic chemotherapy or molecular targeted therapy). The project aims to investigate whether the high response rates resulting from the combination of anti-PD-1 and anti-CTLA-4 can improve the efficacy of HCC patients who may be eligible for radical surgery. This is a one-arm, open-label test. The trial plans to recruit 40 subjects. The expected start date for the study is June 1, 2018, and the expected major completion date is December 31, 2022. New drug name: ASLAN001 (Varlitinib) Drug Mechanism: Tyrosine Kinase Inhibitors Research institution: National University Hospital of Singapore Clinical stage: 1/2 stage ASLAN001 (Varlitinib) is a small molecule tyrosine kinase inhibitor against HER1 (EGFR), HER2 and HER4. This is a one-arm, open label study. Dose-exploration studies were performed in patients with advanced/metastatic hepatocellular carcinoma (HCC) who progressed after first-line treatment with sorafenib. The primary objective of this study was to determine the maximum tolerated dose (MTD) of ASLAN001 (Varlitinib) in the study population. After determining the recommended dose, Part 1b of the study will assess the efficacy of ASLAN001 in advanced HCC patients after sorafenib administration. The trial plan recruited 22 subjects. The expected study start date is May 5, 2017. The expected major completion date is May 5, 2019. New drug name: FOLFOXIRI Drug mechanism: N/A Research institution: China Medical University Clinical stage: Phase 2 Neoadjuvant chemotherapy can inhibit tumors, reduce metastasis, inhibit recurrence, and improve long-term prognosis. The trial will evaluate the efficacy and safety of neoadjuvant FOLFOXIRI chemotherapy (irinotecan, oxaliplatin and fluorouracil) in patients with resectable liver metastases from colorectal cancer. The trial plan recruited 30 subjects. The expected study start date is May 1, 2018. The expected major completion date is May 1, 2020. Note: The title map source 123RF Reference materials: [1] ClinicalTrials.gov [2] Company's official website Iridology Camera Health Analyzer Iris Iriscope Iridology Camera:
Iris holographic diagnostics is a kind of knowledge that applies non-drug and natural therapy to analyze and care for the body by analyzing the iris and pupil to analyze and regulate the health condition of the body. In the field of health medicine, it is called non-invasive health testing and non-drug health intervention. It is a traditional clinical health medicine in Europe.
Iris Iriscope Iridology Camera System Functions:
Iridology Camera,Usb Iridology Camera,Iriscope Iridology Camera,Iridology Camera Eye Iriscope Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.syztreatment.com


1. Adjust brightness through either software or switch on handle line, delete the photos, and adjust the focus, quick fixed photos with white balance and high stability of colors. Connects directly with computer without outside power and easy to operate.
2. The software can save the client information and irises also the details of products that you recommend them. After taken the photos, you can analysis the irises and later compare the irises pictures when your client comes back to see their progress. Can print an analysis report. Save the photos according to the date & time you take the photos.3. This machine will help the client know his health condition, including the problem which you have had in the past. The Iridology will be your health counselor, tell you how to keep away from the illness.

Iris Iriscope Iridology Camera Specification:
* Nice appearance and innovative design
* LED illuminator around lens
* Imported lens with plated layer
* 12MP high resolution CCD sensor
* Special DSP image processor, Optical Image Stabilizer
* Single capture button and digital pause capture.
* Adjustable focus to give clear image.
* Auto white balance and contrast adjustment, Color Temperature Filter
* Compatible with iris lens, hair lens.
* Deliver clear and accurate images.
* Easy to operate.
* OS: Windows XP, WIN2000, 2003, Vista, Win7. Win8,win 10
What is the primary benefit of photo iridology?
Not only does it bring clues to your iridology analysis, but it helps to create a better connection with your patient. The patient, who`s naturally curious, is eager to see their own iris. With some simple explanations they can get a basic understanding of your analysis. The connection with your patient strengthens and their understanding of their process improves.
* Iris analysis system: international technology, unique functions.
* Iris analysis system is a medicinal tool that checks the body conditions and prevents diseases from occurring.
* We brought in the advanced iris analysis technology from Germany to lead people to discover sources of illness, and care the body health and spirit in anyways.
* The instrument can show the body conditions of customers and suggest customers the suitable health food, and the plans to care their bodies.